views
New Delhi:The central government has planned to ramp up testing of suspected and high-risk coronavirus cases in the country due to increasing number hotspots in the country.
To meet the high demands, the Indian Council of Medical Research (ICMR), India's apex biomedical research body, has procured 10 lakh Reverse Transcription-Polymerase Chain Reaction (RT-PCR) kits for diagnosis purposes. Seven depots have been established for the supply of reagents and to ensure efficient distribution to government testing laboratories.
In addition, ICMR is also awaiting delivery of 5 lakh rapid antibody test kits.
So what do these kits consist of and what are the differences? News18 attempts to explain below:
What is the Difference between RT-PCR and Rapid Antibody Test?
The Reverse Transcription-Polymerase Chain Reaction (RT-PCR) test can be described simply as extracting, amplifying and quantifying. It involves the extraction of the RNA of the virus by converting into DNA using a reverse transcription process and then amplification of those DNA using polymerase chain reaction. In simpler terms, it means making thousands and millions of DNA copies and then quantifying them and is essential to help medics detect the presence of the virus and diagnose better.
A rapid antibody test is a blood-based test which effectively checks the blood serum to check if a person has been exposed to a pathogen and if it contains antibodies that a person's body may have produced to fight the pathogens, called an antigen. Antigens are foreign substances that are recognised by the body and an immune response kicks in to fight against the antigen.
Which test is India using to confirm Covid-19 cases?
Currently, the RT-PCR test is being used for confirming cases of Covid-19. Even though both RT-PCR and rapid antibody tests are not 100 per cent accurate, the RT-PCR tests are known to give more rigorous and more accurate results. However, the RT-PCR tests take about 4-6 hours to confirm whether a sample is positive or negative for the virus.
Antibody tests are used for mass screening in potential clusters where there is a fear of community infection. The rapid antibody tests detect antibodies and not the presence of the virus itself. If antibodies are detected in a patient, the samples are collected to run an RT-PCR test for confirmation. A rapid antibody test takes between 20-40 minutes to show results.
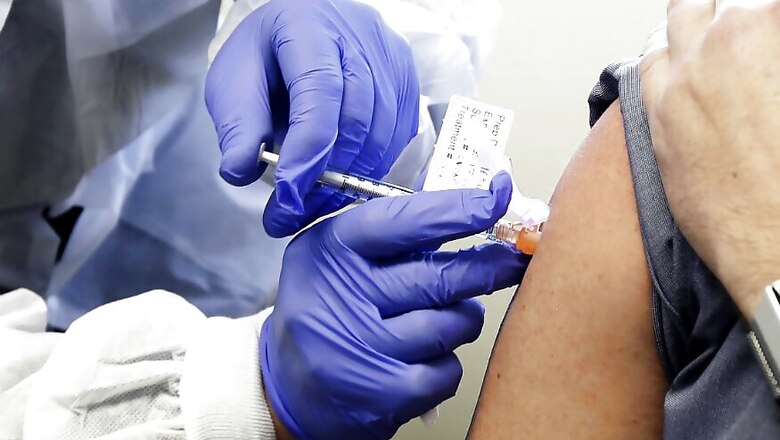
What is Inside the Coronavirus Testing Kits?
An RT-PCR kit consists of all the tools that are required to conduct a test for coronavirus. Dr A Velumani, managing director, of Thyrocare Technologies Limited, compared the components of the kit to ingredients used for making food. “Like we use different ingredients to make food, there are different components in the kit that help us to identify the presence of the virus.”
One kit comprises components that are enough to perform 100 tests, Dr.Velumani said. “It consists of positive controls, RNA extraction kit, RNA amplification kit and a quantifying kit. These are the major components. Probes and primers and buffer solution are the other components.”
Fluorescent DNA binding dyes called ‘probes’ help in the detection of the virus. Primers are used to catalyse the reactions in the RT-PCR test. Positive controls are mostly the freeze-dried material of the virus that is provided in a vial to make sure the test is right.
Components in a rapid antibody test kit vary depending on the kind of rapid test being used. If a point-of-care test is in use, it will come with a small device for pricking a finger for obtaining a blood sample. The rapid tests also come in the form of a home pregnancy test kit. For this test, one drop of serum is dropped in the control bank and the result shows whether the IgM antibodies are present or both IgM and IgG antibodies are present. IgM antibodies are produced in the first 4-7 days after pathogens enter the body while IgG antibodies can be detected 12-14 days after the pathogens have entered the body.
Another form of the rapid test involves two devices, a cartridge-like device that needs to be inserted into a small machine or analyser. The blood serum is drawn and dropped into the cartridge and the analyser gives the results.
What is the difference between kits used by the government and private labs?
According to ICMR scientists, government labs are using in-house testing kits for screening and are sourcing components for confirmation from different institutes that have custom synthesised reagents.
Private labs are using ready-to-use kits made and distributed by domestic manufacturers that have been approved by ICMR.

















Comments
0 comment