views
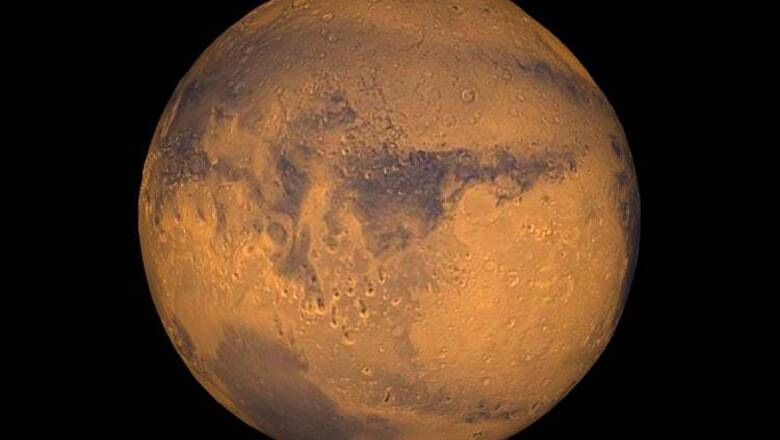
Washington: A team of scientists has offered an explanation of the "missing" carbon on Red Planet, suggesting that it may have escaped into the atmosphere owing to the strong ultraviolet (UV) rays from the Sun.
They suggest that 3.8 billion years ago, Mars might have had a moderately dense atmosphere.
Such an atmosphere -- with a surface pressure equal to or less than that found on Earth -- could have evolved into the current thin one.
"Our paper shows that transitioning from a moderately dense atmosphere to the current thin one is entirely possible,” says postdoctoral fellow Renyu Hu from California Institute of Technology (Caltech).
The solar wind stripped away much of Mars' ancient atmosphere and is still removing tons of it every day.
There are two possible mechanisms for the removal of the excess carbon dioxide.
Either the carbon dioxide was incorporated into minerals in rocks called carbonates or it was lost to space.
One way carbon dioxide escapes to space from Mars' atmosphere is called sputtering, which involves interactions between the solar wind and the upper atmosphere.
NASA's MAVEN (Mars Atmosphere and Volatile Evolution) mission has yielded recent results indicating that about about 100 grams of particles every second are stripped from today's Martian atmosphere via this process.
Sputtering slightly favours loss of carbon-12, compared to carbon-13, but this effect is small.
The Curiosity measurement shows that today's Martian atmosphere is far more enriched in carbon-13 -- in proportion to carbon-12 -- than it should be as a result of sputtering alone, so a different process must also be at work.
Hu and his co-authors identify a mechanism that could have significantly contributed to the carbon-13 enrichment.
The process begins with ultraviolet (UV) light from the Sun striking a molecule of carbon dioxide in the upper atmosphere, splitting it into carbon monoxide and oxygen.
Then, UV light hits the carbon monoxide and splits it into carbon and oxygen.
Some carbon atoms produced this way have enough energy to escape from the atmosphere, and the new study shows that carbon-12 is far more likely to escape than carbon-13.
There are three naturally occurring isotopes of carbon: 12, 13 and 14.
Modeling the long-term effects of this mechanism, the researchers found that a small amount of escape by this process leaves a large fingerprint in the carbon isotopic ratio.
That, in turn, allowed them to calculate that the atmosphere 3.8 billion years ago might have had a surface pressure a bit less thick than Earth's atmosphere today.
"This solves a long-standing paradox,” added Bethany Ehlmann of Caltech and NASA's JPL in a paper published in the journal Nature Communications.

















Comments
0 comment