views
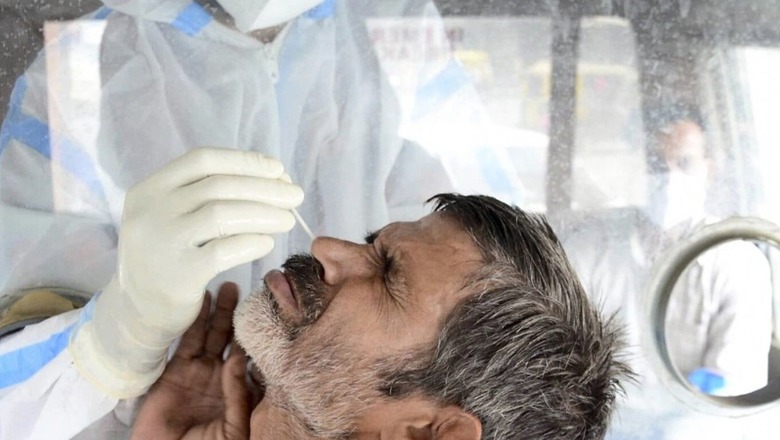
Amid concerns over a possible third wave of the coronavirus pandemic and to speed up the vaccination process in the country, an expert panel of India’s central drug authority has recommended giving an approval to Bharat Biotech to carry out a study on interchangeability of its Covaxin and the under-trial adenoviral intranasal vaccine candidate BBV154. However, the Hyderabad-based pharmaceutical firm was asked to remove the word “interchangeability” from the study title and submit a revised protocol for approval.
As it is given intranasally, the vaccine if approved may also be easier to inoculate, especially for those who have a fear of needles.
WHAT Bharat Biotech SAYS
Talking to a media TV channel, Bharat Biotech MD Dr Krishna Ella had in April spoke about the possibility of a nasal vaccine for coronavirus and explained, “Injectible vaccines only protect up to lower lung, upper lungs and nose are not protected. People vaccinated may get an infection. But the vaccine will prevent you from hospitalisation. You might get a fever for 2-3 days. But mortality will be reduced.”
Further emphasising that Bharat Biotech could be the first one in the world to come up with a nasal Covid-19 vaccine, he had said, “If regulators help, we will be the first although we have competition from the US and China.”
Sharing a study of the intranasal vaccines under clinical trials, Bharat Biotech’s Dr Raches Ella had in July tweeted that “BBV154 is an intranasal Covid-19 vaccine that may overcome the shortfalls of intramuscular vaccines”.
BBV154 is an intranasal COVID-19 vaccine that may overcome the shortfalls of intramuscular vaccines. Perhaps the ideal mode of protection (against disease and infection) might require IgA (mucosal) + IgG (systemic) + Memory B/T cells.
If achieved, we may limit transmission. pic.twitter.com/TfR72lAVuw
— Dr. Raches Ella (@RachesElla) July 26, 2021
Krishna Ella had also shared a data and asked, “Can mucosal (intranasal) vaccine be a gamechanger for SARS-CoV-2”.
Can mucosal (intranasal) vaccine be a gamechanger for #SARSCoV2Given their remarkable allure to block #COVID19 transmission we do need on priority an all hands on deck approach to manufacturing
The case very well explained below https://t.co/tf6n7bWyNQ@WHO @ICMRDELHI pic.twitter.com/4gTgYx5paW
— Dr. Sangita Reddy (@drsangitareddy) July 27, 2021
HOW NASAL VACCINE WORKS?
Nasal vaccines, which are under trial, will block transmission of the SARS-CoV-2 virus in body through nose and help control the Covid-19 pandemic
“The currently available vaccines against Covid-19 are very successful, but the majority of the world’s population is still unvaccinated and there is a critical need for more vaccines that are easy to use and effective at stopping disease and transmission,” Paul McCray, a professor of at University of Georgia, US had said in a study published in July.
Currently, the Covid-19 vaccines available in India are all intramuscular vaccines which ensure that the infection will not become severe after inoculation. The experts believe that a combination of an intramuscular Covid-19 vaccine and a nasal vaccine can be a “gamechanger” for the country to fight against Covid-19.
Besides Bharat Biotech, the other nasal vaccines which are under trial are – Oxford University, Altimmune, University of Hong Kong, Meissa Vaccines, Codagenix and Cuba’s Centre for Genetic Engineering and Biotechnology.
It is being said that nasal vaccine will help to prevent the severity of Covid-19 as it will actually block the coronavirus infection from entering through nose.
Read all the Latest News, Breaking News and Coronavirus News here.

















Comments
0 comment