views
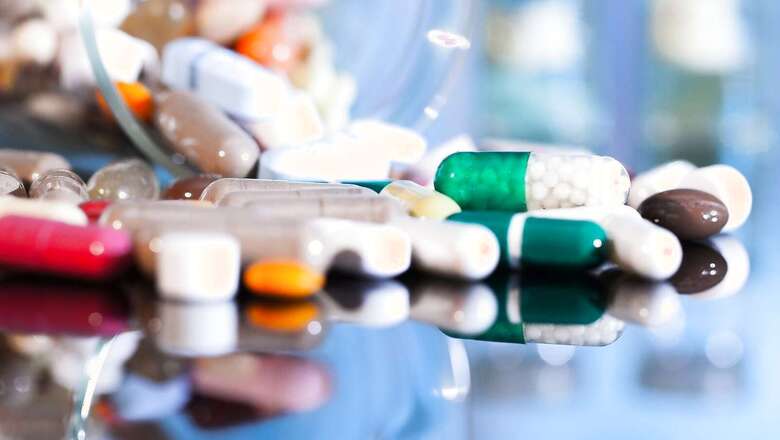
Cocktail medicines, including D’Cold Total, Crocin Cold & Flu, Piriton and Saridon, may not face a ban as the expert panel has recommended continuation of retail sales based on a few conditions, News18.com learnt.
The Central government plans to ban certain fixed drug combinations (FDC) or cocktail medicines which combine more than one drug in a single pill. Cocktail drugs have been under the scanner as a lax regulatory framework allowed several unscientific combinations to flood the market, and there are fears that this may increase drug resistance among people.
Overall, the Central Drugs Standard and Control Organisation (CDSCO) had made a list of 19 FDCs which were deemed as “irrational combinations”. The list has been reviewed by the expert committee constituted under the chairmanship of Dr M S Bhatia, professor and head, of the Department of Psychiatry, University College of Medical Sciences.
According to the report submitted by the committee to the CDSCO and the ministry of health and family welfare, accessed by News18.com, five FDCs that have been proved rational by the manufacturers could escape the ban.
THE REASONS, RECOMMENDATIONS
Taking them out of the ban list, the panel has directed makers of D’Cold Total, Crocin Cold & Flu and others to generate safety and efficacy data on the use of the combination of three drugs, including paracetamol, caffeine, and phenylephrine.
It has recommended conducting phase four clinical trials.
As per the report, the maker of D’Cold Total, Reckitt Benckiser, attended the meetings held by the committee, apart from Crocin maker GSK Asia and Akums Drugs & Pharmaceuticals that also manufactures similar products.
“The committee noted that the FDC is approved in the UK, USA, Germany, Hong Kong, Australia, New Zealand, and Singapore,” said the report by the panel.
“After detailed deliberation, the committee recommended continued manufacturing and marketing of the FDC with the condition to generate safety and efficacy data by way of conducting Phase IV clinical trial.”
Similarly, the drug combination of popular cough syrup Piriton (manufactured by GSK Pharmaceuticals) and Cipla’s Cofton – Chlorpheniramine Maleate, Ammonium Chloride and Sodium Citrate – has been cleared for retail sales, but on certain conditions.
The panel has asked the companies to “modify the prescribing information” by clearly mentioning the dosing schedule for adults and children, keeping in view the decided dose range without exceeding the maximum permissible dose.
Another combination to treat cold and flu – using caffeine, chlorpheniramine maleate, paracetamol and phenylephrine – has been given a go-ahead to continue marketing. The category includes medicines such as Micro Lab’s Dolo Cold and Glenmark’s Alex Cold.
According to the panel’s report, the committee noted that “the FDC is approved in Singapore, New Zealand, and Israel”.
The panel allowed the marketing of the cocktail medicine following certain conditions such as it “shall be sold by retail on the prescription by registered medical practitioner and its package insert should mention caution for patients suffering from cardiovascular diseases”.
“The committee also recommended conducting a randomized comparative, Phase IV clinical trial comparing the FDC with the individual ingredients present in the FDC.”
SARIDON, ANTI-DEPRESSION DRUGS OUT OF LIST
The popular combination of paracetamol, caffeine and propyphenazone, which is used to manufacture the headache relieving pill Saridon, has also been cleared. The committee noted that the FDC is globally approved in 31 countries, including the European Union, Italy, Spain, Hungary and Netherlands. The panel has recommended selling the drug only on prescription and the dose should not exceed five-seven days.
Here also, the panel suggested conducting an active post-marketing study to generate safety and efficacy data.
An anti-depression medicine combination with diazepam and imipramine is sold under various brand names including Tancodep and Prazep. Both the drugs have also been given a go-ahead.
“The FDC shall be indicated about co-morbid anxiety conditions and duration of the treatment should not exceed six-eight weeks,” the panel recommended.
Read all the Latest News India and Breaking News here
















Comments
0 comment