views
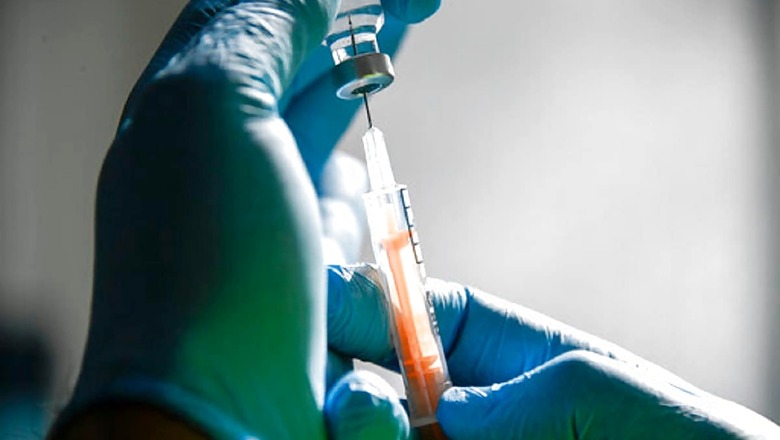
Biological E Limited (BE) has successfully completed the Phase I/II clinical trial of its COVID-19 subunit vaccine candidate in India with promising results and received the approval to start the Phase III clinical trial from the Central Drugs Standard Control Organisation (CDSCO) – Subject Expert Committee (SEC). BE started the Phase I/II clinical trials of its COVID-19 Vaccine candidate in the second week of November 2020, the vaccine maker said in a press release.
The Phase III clinical study will be conducted in 15 sites across India and it will evaluate the immunogenicity and safety of Biological E’s SARS-CoV-2 COVID-19 vaccine for protection against the disease in about 1,268 healthy subjects in the age range of 18 to 80 years. It is intended to be part of a larger global Phase III study.
BE’s vaccine candidate includes an antigen developed by the Texas Children’s Hospital Center for Vaccine Development and in- licensed from BCM Ventures, Baylor College of Medicines integrated commercialization team, along with Dynavax Technologies Corporations advanced adjuvant CpG 1018TM. The Coalition for Epidemic Preparedness Innovations (CEPI) and the Biotechnology Industry Research Assistance Council (BIRAC) have provided support for the Phase I/II clinical trials and also for the upcoming Phase III trial of this vaccine candidate, it said.
BE’s Phase I/II clinical trial evaluated the safety and immunogenicity of the vaccine candidate, consisting of the Receptor Binding Domain of the Spike Protein of SARS-CoV-2 at three-dose level adjuvanted with CpG 1018 plus alum, in about 360 healthy subjects in the age range of 18 to 65 years. The vaccination schedule consisted of two doses for each study participant, administered via intramuscular injection 28 days apart.
BEs novel Covid-19 vaccine was found to be safe and well tolerated and immunogenic, the release said. Mahima Datla, Managing Director, BE, said “We are delighted with the success of the Phase I/II clinical trials of our COVID-19 vaccine candidate.
“The results of these clinical trials are very positive and promising. We believe that our vaccine candidate will become another effective global COVID-19 vaccine as we move forward into Phase III clinical trials.”
(With inputs from PTI)
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.
















Comments
0 comment