views
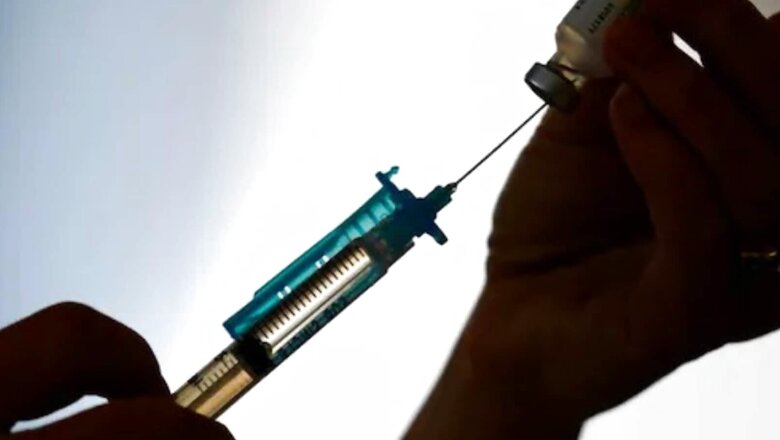
The Phase 2/3 human trials of the country’s first homegrown mRNA vaccine developed by Pune-based Gennova Biopharmaceuticals have been completed on Tuesday. The organisation is now in the process of submitting data to the national regulatory authority. Along with submitting the data, there are plans afoot to test the vaccine in the pediatric population initially in a phase 2 trial.
According to an Indian Express report, the Drug Controller General of India (DCGI) had approved Phase II and Phase III study protocols for HGCO19, India’s first mRNA-based COVID-19 vaccine. The firm had submitted the interim clinical data of the Phase 1 study then to the Central Drugs Standard Control Organisation.
What is gennova Vaccine?
Emcure Pharmaceuticals says that Gennova, its subsidiary, is “India’s only company to deploy the mRNA platform to develop a vaccine” against the novel coronavirus. It has developed its candidate, known as HGC019, in collaboration with the US-based HDT Biotech Corporation, starting work on the vaccine right after the Sars-CoV-2 genome was published in January 2020.
It was reported in August 2021 that India had okayed further human trials of the Gennova vaccine after the shot was found to be safe and effective in initial studies, demonstrating “safety, immunogenicity and neutralisation antibody activity in rodents and non-human primate models”. Clinical trials are partly funded by India’s Ministry of Science and Technology, reports said.
Gennova Biopharmaceuticals working on an Omicron-specific COVID-19 vaccine
India’s Gennova Biopharmaceuticals is also working on an Omicron-specific mRNA COVID-19 vaccine candidate, it told Reuters. Reportedly, the product could be ready in a month or two. “The Omicron-specific variant of the vaccine is under development and will be ready for human clinical trials, subject to regulatory approvals,” a company spokesperson said in a text message. The source, who did not want to be named as the information was private, said the product might need a small trial in India before it could be rolled out.
What is an MRNA Vaccine?
These vaccines belong to the category of nucleic acid vaccines, which use genetic material from a disease-causing virus or pathogen to trigger an immune response against it within the body. All vaccines seek to expose the body to the active part of the pathogen that enables it to infect humans so that the immune system recognises it and produces antibodies against it in the future. How traditional vaccines achieve this is by inserting a part of the virus itself — either weakened or inactivated — into the body.
How many vaccines India has approved so far?
India has so far approved at least six vaccines that can be manufactured locally but only two — Covishield and Covaxin —have been administered to over 99% of Indians. Globally, mRNA vaccines have been at the vanguard of inoculation programmes in the United States and Europe because they exploit recent advances in molecular biotechnology and are said to be quicker to manufacture than older, well-established vaccine design principles.
What are the limitations of mRNA vaccines?
A limitation of the mRNA vaccines, or those made by Pfizer and Moderna, was that they were required to be stored in sub-zero conditions — a tough proposition in a country where such a degree of refrigeration is limited in availability. However, the prospective Gennova vaccine can be stored in ordinary refrigerators, the makers of Gennova have claimed earlier.
Read all the Latest India News here



















Comments
0 comment