views
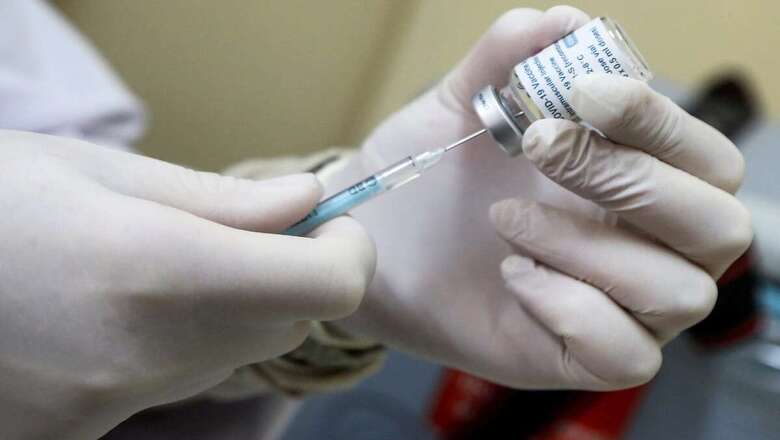
After Oxford-Astrazeneca’s Covid-19 vaccine, Johnson & Johnson’s Janssen vaccine has hit roadblocks over reports of blood clotting. The US on Tuesday recommended a pause in administration of the Johnson & Johnson COVID-19 vaccine after reports of potentially dangerous blood clots. In a joint statement Tuesday, the Centers for Disease Control and Prevention and the Food and Drug Administration said it was investigating clots in six women in the days after vaccination, in combination with reduced platelet counts.
More than 6.8 million doses of the J&J vaccine have been administered in the US.
The Johnson & Johnson company is now delaying the rollout of its coronavirus vaccine in Europe after US regulators announced a pause on the vaccine administration. “We have been reviewing these cases with European health authorities,” Johnson & Johnson said. “We have made the decision to proactively delay the rollout of our vaccine in Europe.” Australia too has refused to add J&J vaccine to its armory.
Several countries in the 27-nation bloc have limited the AstraZeneca vaccine to older age groups, which are more at risk from serious illness when infected with COVID-19 after reports of blood clots. J&J and AstraZeneca vaccines are made with the same technology.
Many leading COVID-19 vaccines train the body to recognize the spike protein that coats the outer surface of the coronavirus. But the J&J and AstraZeneca vaccines use a cold virus, called an adenovirus, to carry the spike gene into the body. Johnson & Johnson uses a human adenovirus to create its vaccine, while AstraZeneca uses a chimpanzee version.
While J&J vaccine still awaits nod for use in India, AstraZeneca’s Covishield is one of the two vaccines currently being given in India.
How dangerous are these blood clots?
Cheaper and more easily stored than other Covid-19 vaccines, and hailed to be safe and 79% effective at preventing symptomatic Covid-19, AstraZeneca vaccine has been suspended in people under 60 years of age in many countries including South Korea and the Philippines. Known as cerebral venous sinus thrombosis (CVST), it is a very rare and specific type of blood clot in the brain occurring together with low levels of platelets (thrombocytopenia) following vaccination. Noting the condition as “unusual”, the French Medicines Agency (ANSM) told AFP, “This thrombosis of large veins is unusually located in the brain, and even more rarely in the digestive tract.”
In the Indian context, the country should have reported 320 cases of blood clot associated with administering of Covishield vaccine by now if the European levels of risk calculated for AstraZeneca were to apply to the country, according to eminent scientist Gagandeep Kang. She termed it a “very small risk” with absolutely no need for apprehension. Kang, Professor at the Christian Medical College, Vellore, said that the European levels of risk of blood clots from the AstraZeneca vaccine are 1 out of 100,000 according to the European Medicines Agency (EMA) and 1 out of 250,000 as reported by the British Health Regulator.
“There are bound to be a few or several cases of people who have had blood clots after getting a jab. That is to be expected. What we need to know is whether these blood clots were accompanied with a low platelet count,” Kang told The Wire in an interview. She said the government should set up an inquiry to look into this matter in a time-bound manner and make its report fully public. According to the virologist, if the European-level calculations were to apply to India, the country would have a total of 3,000 cases of blood clots out of its total target of 300 million people to be vaccinated.
According to Kang, out of the 80 million doses of the Astrazeneca the vaccine is already given, India should have 320 cases. She added that this is a “very small risk” and there is absolutely no need for apprehension. However, she said the exact numbers of such cases are not known currently.
(With PTI inputs)
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.
















Comments
0 comment