views
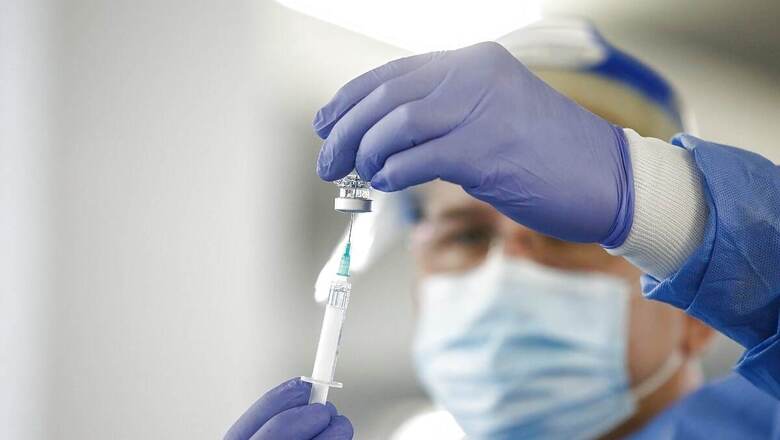
Amid reports of shortage of Remedsivir in states, head of the national Covid task force, Dr VK Paul, has said that the drug should be used only in hospitals and not at pharma shops. He added that it is for the people admitted at hospital and not for those in home. The doctor asked people to aggressively join a ‘mask movement’, “else the virus won’t stop”.
Paul further suggested people to have Chyawanprash, Tulsi kadah and turmeric milk as “traditional methods like Ayurveda work best for immunity boosting”. Following Centre’s decision to fast track approvals for foreign-produced vaccines against coronavirus, the COVID task force head said India will witness a quantum increase in vaccinations during Tika Utsav.
The path has been opened up for increased manufacturing of vaccines and ball is now in the court of the companies, he said. The Centre on Tuesday decided to expedite the process of approvals for COVID-19 vaccines, that have been produced abroad and granted Emergency Use Authorisation (EUA) in other countries.
“The government will examine the decision on pricing of imported vaccines. It will wait for prices being offered by vaccine makers and pricing issues will be examined against the present framework,” Paul said in an interview to CNBC-TV18.
He further said, “Vaccine availability will improve from July. We will be entering a phase of ‘generous’ availability where there will be no vaccine shortage for priority groups.”
The government is engaged with the Pune-based Serum Institute of India and Bharat Biotech in Hyderabad to assist them in ramping up production of vaccines, he said. “The government is also considering financial assistance to SII and Biotech,” he added. The SII, world’s largest vaccine manufacturer has partnered with Oxford-AstraZeneca to produce Covishield, while Bharat Biotech is making Covaxin, both of which have been approved by the government of India.
On the issue of bridging trials, he said, “It can happen simultaneously with inoculations. The need for phased clinical trials is done away with and a bridging study can be done after the vaccine is made available in India.
In its notification on Tuesday, the Centre said, “Vaccination is one of the critical pillars of COVID control and management strategy adopted by the Centre. Presently two vaccines i.e. Covaxin by Bharat Biotech International Limited (BBIL) and Covishield by Serum Institute of India (SII), have received Emergency Use Authorization (EUA) from the National Regulator (Drugs Controller General of India).”
The Centre’s decision came after an expert panel recommended that COVID-19 vaccines which have been developed and are being manufactured in foreign countries and which have been granted emergency approval for restricted use by authorities in the US, Europe, the UK, Japan or which are listed in the WHO Emergency Use Listing may be granted emergency use approval in India. The expert panel also mandated the requirement of post-approval parallel bridging clinical trial in place of conduct of local clinical trial.
“This decision will facilitate quicker access to such foreign vaccines by India and would encourage imports including import of bulk drug material, optimal utilization of domestic fill and finish capacity, etc., which will in turn provide a fillip to vaccine manufacturing capacity and total vaccine availability for domestic (use),” the ministry said. Currently, two vaccines — Covaxin by Bharat Biotech and Covishield by Serum Institute of India (SII) — are being used for inoculation in India.
India’s drug regulator has also granted permission for the restricted emergency use of the Russian COVID-19 vaccine Sputnik V with certain conditions on Monday.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.

















Comments
0 comment