views
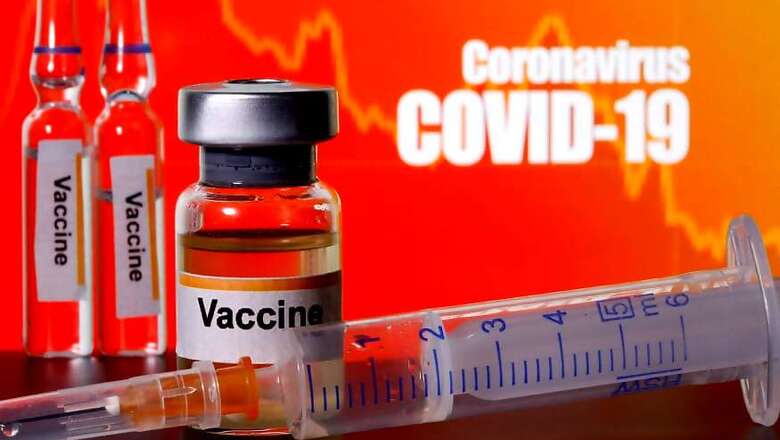
Officials who appeared before a parliamentary panel on Friday said no vaccine for coronavirus will be ready before next year, sources told CNN-News18.
The Parliamentary Standing Committee on Science and Technology, Environment and Climate Change met for the first time today since the lockdown due to COVID-19 was enforced on March 25. The panel headed by Congress leader Jairam Ramesh held its first meeting since Parliament was adjourned on March 23, ahead of its scheduled time.
Presentations were given by the Department of Science and Technology, Department of Biotechnology, Council of Scientific and Industrial Research (CSIR) and the principal scientific adviser to the government before the Parliamentary Standing Committee on Science and Technology, Environment and Climate on the Centre's COVID-19 preparedness.
Sources said it was conveyed to the panel that a vaccine for COVID-19 could be available only by early next year.
Rajya Sabha Chairman M Venkaiah Naidu said the delay in the resumption of parliamentary committee meetings was forced by circumstances beyond control.
"I am glad that Department Related Parliamentary Standing Committees have resumed functioning, three and half months since the last sitting of Parliament on March 23rd," he wrote on Twitter.
Naidu said everyone was keen on resumption of work by these committees, "but the delay was forced by circumstances beyond our control".
"Corona pandemic did cast a shadow on the working of these committees, which function on behalf of the Parliament," the vice president said.
Naidu said all possible measures were taken to enable the committee meetings by complying with the norms of social distancing, wearing of masks etc. "I am hopeful these committees would now go about examining important issues concerning respective domains," he said.
Urging Naidu for the holding of virtual meetings, Ramesh, in a tweet, said, "I would still request you Sir to allow virtual meetings given that Parliament is unlikely to meet for the next month at least."
The sources said the other members present at the meet also sought virtual deliberations.
Indian Council of Medical Research (ICMR) Director General Dr Balram Bhargava had earlier 2 written to principal investigators of select medical institutions and hospitals to fast-track human clinical trial approvals for the vaccine candidate 'Covaxin' being developed in collaboration with Bharat Biotech.
After criticism by scientists and experts, the ICMR in a statement on July 4 said the letter was meant to cut unnecessary red tape, without bypassing any necessary process, and speed up recruitment of participants.
The Drugs Controller General of India (DCGI) has permitted two vaccines -- one developed by the Bharat Biotech International Limited in collaboration with ICMR and another by Zydus Cadila Healthcare Ltd -- to go in for phase 1 and 2 human clinical trials.
The sites for phase 1 and phase 2 clinical trials have been finalised and the trials are yet to begin.
(With inputs from PTI)


















Comments
0 comment