views
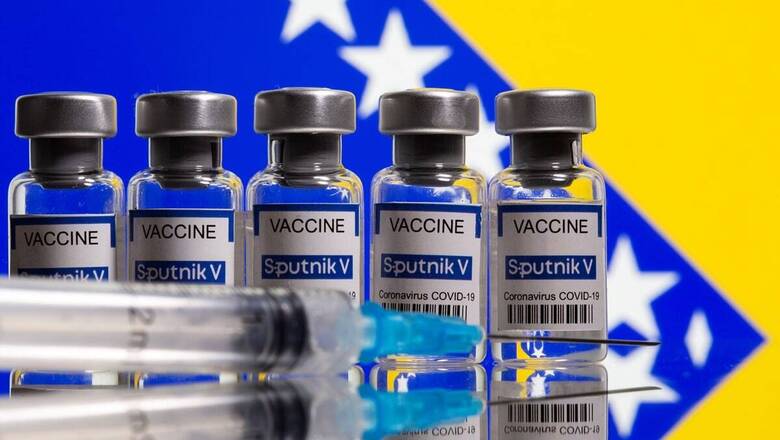
The emergency use authorisation from the Drugs Controller General of India (DCGI) for Sputnik V has paved the way for import and use of the Russian vaccine against COVID-19 for restricted use in India. The big question now is the production process and when and how will Sputnik V reach vaccination centres in India.
Government sources have told CNN News18 that it is exploring the possibility of importing Sputnik V from Russia by the following three mechanisms on getting the go-ahead from the central laboratory seeking manufacturing licence from DCGI.
– Bulk import
– Technology transfer and then production of vaccine in India
– Import in volumes rather than vials. Then fill and finish in India
The production process would take at least two-three months for the Sputnik-V vaccine to be made available. Hence, the first locally produced batch of Sputnik V is likely to be available by June.
The Russian Direct Investment Fund (RDIF) has partnered with Hyderabad-based Dr. Reddy’s Laboratories for clinical trials and distribution of the first 100 million doses in India. In a statement, Dr. Reddy’s Managing Director GV Prasad said on Tuesday, “We are very pleased to obtain the emergency use authorisation for Sputnik V in India. With the rising cases in India, vaccination is the most effective tool in our battle against COVID-19. This will enable us to contribute to our nation’s effort of vaccinating a significant proportion of our population.”
The Russian vaccine has an efficacy of 91.6%, according to a publication in The Lancet, and has now been approved for use in 60 countries. It will be India’s third vaccine after Covishied and Covaxin that were granted ‘conditional approval’ from DCGI on January 3.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.

















Comments
0 comment