views
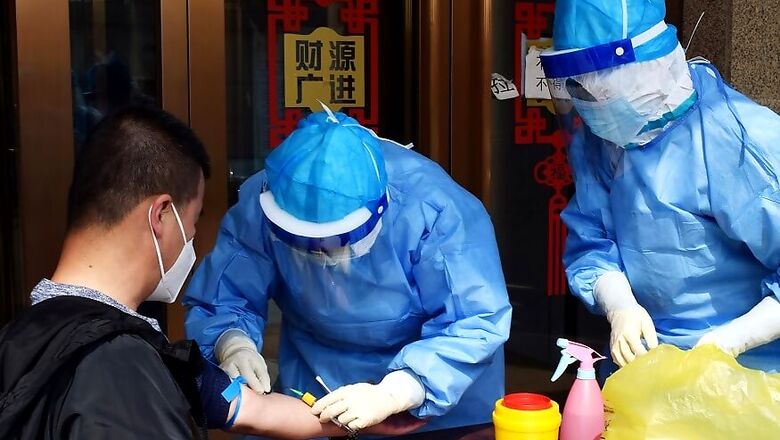
Medical device maker Danaher Corp said on Monday its COVID-19 blood test for detecting if a person had ever been infected with the new coronavirus received emergency use clearance from the U.S. Food and Drug Administration.
The company's unit Beckman Coulter said it had shipped the antibody tests to nearly 400 U.S. hospitals and laboratories, and has ramped up production to deliver more than 30 million tests a month.
Antibody tests, which can indicate a certain degree of immunity in those who have had the virus, are seen crucial in safely reopening economies after weeks of lockdowns.
High demand for the test has led to the entry of several fraudulent kits, prompting the FDA to tighten its rules over the tests.
Danaher said its antibody test has a specificity rate of 99.6% and 100% sensitivity, suggesting very few chances of false positives and no false negatives.
Abbott Laboratories' antibody test has a specificity rate of 99.9%, while the rate stands at 99.8% for Roche Holding AG's test. Both companies have received the FDA's emergency use authorization for their tests.
Danaher also said it has begun distribution of its antibody test in other countries that accept the U.S. FDA's clearance.



















Comments
0 comment