views
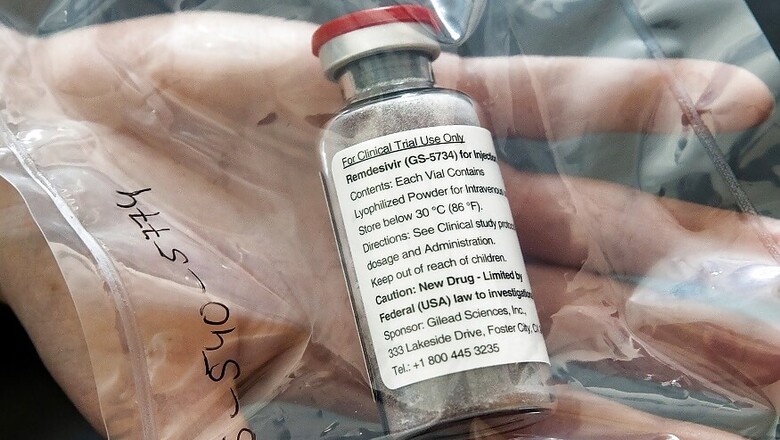
Jubilant Life Sciences on Monday said it has received drug regulator DCGI approval for generic version of antiviral drug remdesivir 100 mg/vial for restricted emergency use in India for treatment of severe COVID-19.
The company's subsidiary Jubilant Generics has received the approval from Drug Controller General of India (DCGI) to manufacture and market investigational drug remdesivir 100 mg/vial (lyophilized injection), Jubilant Life Sciences said in a late night filing to BSE.
Jubilant's remdesivir will be marketed under the brand name 'JUBI-R' in India and will be made available in 100 mg vials (injectable), it added.
The company will distribute the drug in the Indian market through its network and it will be available by the first week of August 2020, the filing said.
The company said it is focused on quickly making this drug available in India in required quantities and at affordable prices.
Jubilant Life Sciences, however, did not provide any details about the price of the drug.
In May 2020, Jubilant entered into a non-exclusive licensing agreement with Gilead Sciences Inc that granted it the right to register, manufacture and sell Gilead's investigational drug remdesivir in 127 countries, including India












Comments
0 comment