views
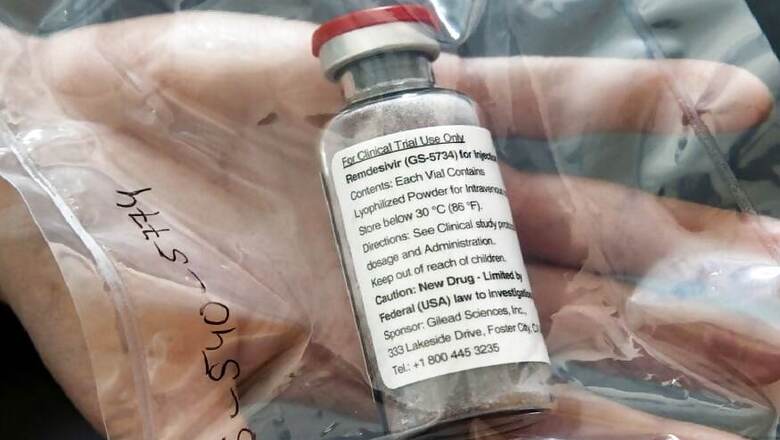
The government said on Tuesday it has approved Gilead Sciences Inc's antiviral drug remdesivir for emergency use for five doses in treating COVID-19 patients.
Remdesivir, which is administered intravenously in hospital, is the first drug to show improvement in COVID-19 patients in formal clinical trials and is at the forefront of the battle against COVID-19, which has no approved treatment or vaccine.
The drug was granted emergency use authorization by the US Food and Drug Administration last month and has received approval by Japanese health regulators. The drug is being administered in some countries under compassionate use rules.
"(Remdesivir) approved on June 1 under emergency use with condition for five dose administration," the Drugs Controller General of India said in an email statement.
The approval comes a day after the US drugmaker reported that remdesivir showed modest benefit in patients with moderate COVID-19 given a five-day course, while those who received it for 10 days in the study did not fare as well.
The drug has been approved for the treatment of adults and children with severe COVID-19, according to a report on Tuesday.
The regulator has decided against extending the use of the drug to 10 days, based on existing evidence presented to it at the time of approval, according to the paper. Gilead did not respond to an email seeking further details.
The company signed non-exclusive licensing pacts last month with five generic drugmakers based in India and Pakistan, including Cipla Ltd and Jubilant Life Sciences Ltd, to expand supply of the drug.
As of Tuesday, India has 1,98,706 cases of coronavirus and has recorded 5,598 deaths, health ministry data showed https://www.mohfw.gov.in.
Governments are racing to bolster supplies of remdesivir, with European and South Korean authorities vying for the potential COVID-19 treatment.
















Comments
0 comment