views
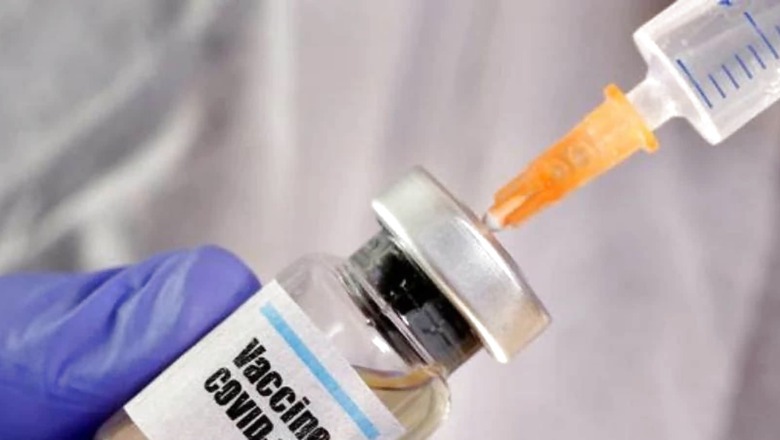
Recently media reported the INSACOG (Indian SARS-CoV-2 Genomic Consortium) recommending booster doses of COVID-19 vaccines for population groups in the country who either have a high risk of severe disease if infected or a high risk of severe exposure to the virus. They quoted the group as proposing such doses for all persons over the age of 40 years. INSACOG later clarified that there was only a discussion among the experts, but no recommendation or suggestion. Clearly, such a recommendation would have been beyond the government’s mandate to INSACOG.
Nevertheless, the booster debate is now active in scientific circles and the media. Several independent experts have suggested early completion of full vaccination to all eligible adults and further administration of booster doses to all persons above 60 years of age. They note the arrival and fast spread of the Omicron variant, while making these recommendations. In their view, providing additional protection to the high risk groups now acquires high priority.
While weighing available scientific evidence, there are several questions which need to be addressed. Who are the people who qualify for boosters? Do all previously vaccinated adults need them? Will one booster suffice? Which are the vaccines that require boosters and at what time after the previous schedule was completed? What is the evidence that boosters will work against the new variant which is suspected of having acquired greater ability to evade immunity directed at the spike protein?
ALSO READ | State Must Not Drag Its Feet and Make Covid Vaccination Mandatory for All Adults
The Science on Boosters
When newly developed COVID vaccines were widely welcomed by a waiting world, there was unfounded expectation that the vaccines would prevent infections by the virus. Unfounded, because systemically administered intra-muscular vaccines evoke a systemic immune response that resists a virus which has entered the respiratory tract but does not prevent the initial infection per se. They help us to quickly and effectively overcome the virus which has infected us, preventing serious illness and death in most cases. To prevent the infection itself, we require secretory antibodies which wash away the virus even as it tries to settle in the mucous membranes of the respiratory tract. Such mucosal vaccines for COVID are being developed and trialled but proven products are not yet available. We do not presently know if they will be shown to be effective in the ongoing clinical trials.
Vaccines are assessed in clinical trials, for efficacy and safety. Each of the presently approved vaccines has passed that test. Duration of the developed immune response can be assessed only in real life experience after the vaccines are administered to large numbers of the general population. If variants develop later, efficacy of the already licensed vaccines against each variant is assessed both through laboratory tests and population studies. In the laboratory, scientists study the ability of the antibodies that were developed by previously vaccinated persons to neutralise the new variant. In the general population, the incidence of ‘breakthrough’ infections is studied.
Among available vaccines, most have been directed against the spike protein of the virus. The spikes lie on the outer surface of the virus. They are used by the virus to attach itself to the ACE 2 receptor on human cells and prise its way into the cell, where it uses the cell’s genetic material to make many copies of itself. The mRNA vaccines have produced an exuberant response of anti-spike protein antibodies and showed very high levels of clinical efficacy (over 90 per cent) against the original virus. Their levels, however, declined in a few months. The Pfizer-BioNTech vaccine showed a decline from the fifth month after the second dose. The Moderna vaccine, which used a higher dose of the antigen, had a longer duration of efficacy but that vaccine too appeared to require a booster after 6-8 months.
Further, efficacy levels diminished with each new variant, Alpha to Delta. The mRNA vaccines were evaluated in clinical trials at a time when the ancestral virus from Wuhan was the active agent of infection and when the Alpha variant was just emerging. The efficacy levels these vaccines demonstrated against the ancestral virus showed a dip against the Alpha variant. The decline in efficacy was sharper against the Delta. This was because of the number and shape of the spike protein mutations which differentiated them from the original virus whose spike protein configuration was incorporated into the mRNA vaccines. The AstraZeneca virus vector vaccine, also primed against the spike protein, showed such a decline too. Hence the proposal for additional (‘booster’) doses of these vaccines.
The virus vector vaccines appear to have a longer duration of efficacy and may need a booster only after 10-12 months. The duration of efficacy of inactivated virus vaccines (like Covaxin) has not been assessed but the manufacturer says that a booster dose may be required after 6 months. This type of vaccine offers a larger platter of viral antigens to stimulate the immune system. So, the immune response is not spike protein-specific and is therefore not as vulnerable to the emergence of variants with spike protein alterations.
Boosters can be ‘homologous’ in which the same vaccine is repeated or ‘heterologous’ where a vaccine from a different platform is used. From preliminary studies, the latter approach appears to yield a stronger immune response. The big question, as yet unanswered, is whether a booster dose of a spike-specific vaccine which has shown declining efficacy, against variants with new spike mutations, be effective against them? Or will boosted antibody response to some of the old spikes which are still carried, alongside the mutations, overwhelm the variant? We have to wait for more information on this. Vaccine manufacturers are proposing to tweak their mRNA and protein codes of virus vector vaccines to make them more effective against the variants. We do not know how often this may be required. In the meanwhile, the inactivated virus vaccine can be used for boosters without alteration, as it is not spike specific.
Global Trends on Boosters
Where vaccines are in abundant supply, countries are offering boosters to all who were vaccinated 6-8 months earlier. Others are restricting, as per WHO guidelines, to the elderly and immunocompromised persons. Persons with high risk of exposure to the virus are also prioritised for timed boosters in some other countries, irrespective of age or co-morbidities. Such persons include healthcare personnel who are likely to be exposed to many patients with high viral loads.
The delta virus appears to have waned in India, though it may resurface in crowded events involving under-vaccinated population groups. The imminent threat appears to be that of Omicron which has the potential to spread rapidly. Though early indications from gathering global experience are of milder illness, compared to earlier versions of the virus, the impact on elderly persons and immunocompromised individuals is not yet clear. Healthcare personnel, in direct and repetitive contact with many patients, will also be at high risk of breakthrough infections, especially as they would have received both their shots early during January to April 2021. Mass booster dose administration, for all adults, does not appear warranted at present.
Timing of initiating booster doses, and selection of persons at high risk for administering them, will be dependent on the vaccine supplies available at any time. As production volumes of previously licensed vaccines will go up and other domestically manufactured vaccines get regulatory approval, supplies are likely to increase substantially. Boosters can then be undertaken without supply constraints. Till then completion of two dose adult vaccination will probably remain the highest priority for the government. Export of vaccines will also continue, both to meet commercial obligations and to promote global vaccine equity. As the situation seems under control presently, vaccine policy may not change soon. However, if Omicron challenges us with disconcerting speed and spread across the country, protection of high risk groups will become imperative. The coming weeks will decide which way the pendulum swings on boosters, as India warily watches out for a possible Omicron wave.
Professor K. Srinath Reddy, a cardiologist and epidemiologist, is President, Public Health Foundation of India (PHFI). The views expressed in this article are those of the author and do not represent the stand of this publication.
Read all the Latest Opinions here

















Comments
0 comment