views
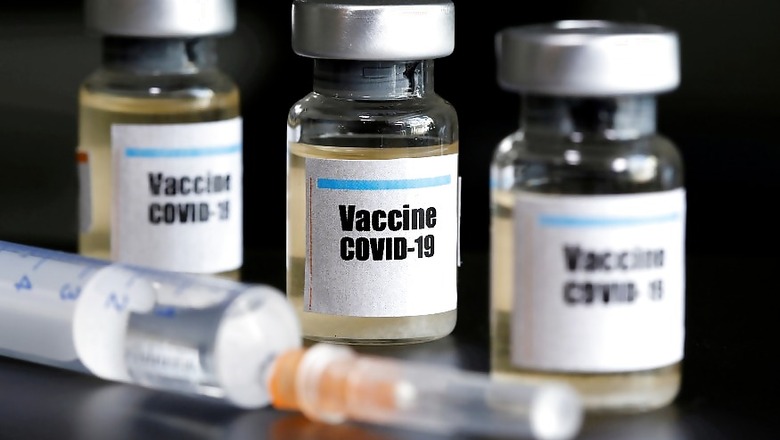
Can a vaccine, whose development began just over two months ago, be rolled out in a span of three months?
This question has been on the minds of many ever since Friday morning after Indian Council of Medical Research (ICMR) Director-General Dr Balram Bhargava said the government has envisaged launching the coronavirus vaccine being developed with Hyderabad-based Bharat Biotech by August 15 after completion of clinical trials.
“BBIL is working expeditiously to meet the target. However, final outcome will depend on the cooperation of all clinical trial sites involved in this project,” read Dr Bhargava’s letter to 13 hospitals chosen for the clinical trials.
The letter’s contents and tone have left the scientific community puzzled. Experts with experience of clinical trials said there seems to be an unusual hurry in the way institutes have been asked to speed up decisions related to approvals for trials.
“In view of the public health emergency due to Covid-19 pandemic and urgency to launch the vaccine, you are strictly advised to fast track all approvals related to initiation of the clinical trial and ensure that the subject enrollment is initiated no later than 7th July, 2020,” the letter read.
“Kindly note that non-compliance will be viewed very seriously. Therefore, you are advised to treat this project on highest priority and meet the given timelines without any lapse,” it further said.
Experts have also questioned the accelerated timeline for launch of the vaccine, , named COVAXIN, and warned of consequences if it is rushed.
“Why are they in such a tearing hurry?” asked Dr Shri Prakash Kalantri, director professor (medicine) of Mahatma Gandhi Institute of Medical Sciences and Medical Superintendent of Kasturba Hospital, Wardha.
“The tone of the letter suggests that they want the institutional ethics committees to sign on the dotted line immediately. Curiously, the institutes chosen as trial sites are not even academic institutions as is the usual practice,” Dr Kalantri said.
Raising questions on a potential conflict of interest, Dr Kalantri said, “ICMR is involved in the trial and it is also supervising and issuing a diktat to the sites of clinical trials.”
While refusing to comment on the specific directions issued in Dr Bhargava’s letter, former ICMR DG Dr Nirmal Kumar Ganguly said that each stage of clinical trial of a vaccine, if followed as per protocol, consumes months even after fast-tracking approvals.
“The vaccine development pathways are very clear. After preclinical stage that involves animal studies, if there are no adverse effects seen, the trials move to human trials phase. In human trials phase, we check for safety, side effects, dosage, immunogenicity and efficacy. Each phase is a time-consuming process and needs participants who have given full consent.”
What has Bharat Biotech claimed so far?
The Hyderabad-based company, which has developed vaccines for H1N1, Rotavirus, Japanese Encephalitis, Rabies and Zika among others, said earlier this week that the Drug Controller General of India has granted it permission to initiate Phase I and II human clinical trials for COVID-19 vaccine.
This came after it submitted results generated from preclinical studies, demonstrating safety and immune response.
“Expedited through national regulatory protocols, the company accelerated its objective in completing the comprehensive pre-clinical studies. Results from these studies have been promising and show extensive safety and effective immune responses,” the company said in a press note earlier this week.
When contacted for a response on the ICMR DG’s letter, the company’s media relations personnel Sheela Panicker declined to comment.
The ICMR, meanwhile, just said that the letter requests the institutes to fast track the vaccine trials and did not comment on queries on whether the vaccine or vaccine data will be launched by August 15.












Comments
0 comment