views
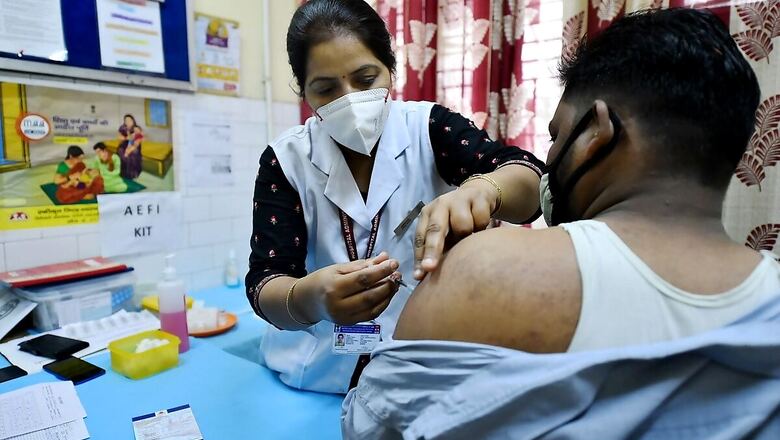
Day after Russia’s Sputnik V received expert panel nod for emergency use in India, shots from other countries are expected to get a fast-track approval as infection rates continue to soar to a record high, authorities said on Tuesday. The move to expedite green signals to vaccines comes at a time when exponential surge in coronavirus cases coupled with sluggish vaccination drive in the population of 1.3 billion people has put immense pressure on the country’s crippling healthcare infrastructure.
India currently has two vaccines available- Oxford-AstraZeneca’s Covishield and Bharat Biotech’s indigenous Covaxin. Russian vaccine Sputnik V on Tuesday got expert panel approval for emergency use in India. With this, India became the 60th country to approve Sputnik V. Sputnik V, backed by the Russian Direct Investment Fund (RDIF), already has production agreements in India to produce 852 million doses. RDIF chief executive Kirill Dmitriev said in a statement that the approval was a “major milestone” after “extensive cooperation” on clinical trials of the shot in India.
The health ministry has said it would expedite the approval of vaccines not made in India to “expand the basket of vaccines for domestic use and hasten the pace and coverage of vaccination”. The jabs will need to have been already been granted emergency-use authorisation by regulators, such as the US Food and Drug Administration, the European Medicine Agency and others in Britain and Japan or the World Health Organisation, the ministry said. A requirement for pre-approval clinical trials would be replaced by post-authorisation trials. This move from the government has given hopes to India’s fight against coronavirus as the fresh wave has crippled the healthcare system across the country.
Other shots which can be expected to be included in India’s basket of shots include Pfizer and Moderna. According to the World Health Organisation, as per data available till 18 February 2021, at least seven different vaccines across three platforms have been rolled out in countries. At the same time, more than 200 additional vaccine candidates are in development, of which more than 60 are in clinical development. COVAX is part of the ACT Accelerator, which WHO launched with partners in 2020.
Here is a quick look at other vaccines which can be expected to be rolled out in India:
Pfizer
Approved by US Food and Drug Administration for emergency use, Pfizer-Biontech vaccines are advised for people aged above 16 and vaccination is completed in two shots, 21 days apart. Over 44 countries, including US, UK, Canada, Australia, Chile and Singapore, have approved the vaccine. Pfizer’s production facility is located in Belgium. With regard to storage and transportation, Pfizer vaccine can be stored at normal freezer temperatures for up to two weeks during transport and at regular fridge temperatures for up to five days.
Moderna
This vaccine too has FDA approval for emergency usage and is advised for people above 18 years of age for Covid-19 protection, with 94% efficacy. Moderna is also given in two doses, 28 days apart. This vaccine needs to be stored in a freezer between -25°C and -15°C. According to CDC, Moderna vaccine vials may be stored in the refrigerator between 2°C and 8°C for up to 30 days before vials are punctured.
Johnson & Johnson
Unlike most of the other vaccines, J&J’s Janssen vaccine is given in a single shot and is authorised by British health regulator. The vaccine is reported to be only 66% efficient against Covid-19 protection. However, the vaccine has hit a roadblock in various countries, including US, and has been called off after reports of blood clotting. Top US health authorities recommended a “pause” in the use of the Johnson & Johnson vaccine “out of an abundance of caution” as they investigate any links between it and blood clots, a regulator said Tuesday.
Novavax
Being manufactured in collaboration with Serum Institute of India, US-based Novavax is another vaccine in pipeline for India. Like most other vaccines, this jab is also given in two doses. Unlike Pfizer and Moderna which require very low temperatures, this vaccine can be stored at normal fridge temperatures, reducing chances of wastage. Novavax has an efficacy of 89.3% in providing protection against the virus and has shown encouraging results in the trials. The vaccine could receive an Emergency Usage Approval by FDA in May and will be expected for use in India by September.
Zydus Cadila
The Gujarat-based pharmaceutical company expects nod on emergency usage of its vaccine ZyCov-D and will be available by August. The chairman of the Zydus group Pankaj Patel had earlier said that the vaccine will be different in the sense that it is DNA plasmid platform vaccine. The Zydus jab will be intradermally through a specific device. It is needle-free, painless administering, Patel had said, adding that side effects will be minimal.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.

















Comments
0 comment