views
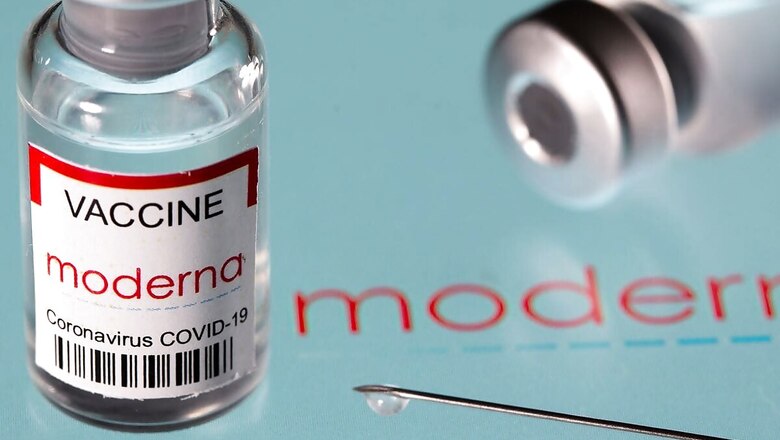
Cipla, a multinational pharmaceutical company headquartered in Mumbai, has sought Drugs Controller General of India nod for importing Moderna’s coronavirus vaccine, sources told CNBC-TV18.
The company submitted an application on Monday seeking approval to import the vaccine, sources further said, adding that DCGI may give green signal on Tuesday itself.
Moderna’s method to protect against Covid-19 relies on messenger RNA (mRNA) to program cells to generate immunity to the coronavirus. This vaccine along with Pfizer are viewed as a preferred choice among wealthy countries, analysts said, based on clinical trial data showing they were more than 90% effective at preventing symptomatic coronavirus.
About 120 million Americans have received a Pfizer or Moderna shot so far with no major safety issue identified.
The United States and European Union are pushing to stock up on even more of the mRNA vaccines. Japan is also working to secure 100 million doses of Pfizer’s shot by the end of June.
The higher cost, production limits and demanding requirements for shipping and storage could limit mRNA-based vaccines’ availability in lower income countries, experts said.
“Right now, (mRNA-based shots) are the Lamborghinis or McLarens of COVID-19 vaccines,” said Dr. Peter Hotez, a vaccine researcher at Baylor College of Medicine in Houston, referring to ultra high-end luxury automobiles.
Read all the Latest News, Breaking News and Coronavirus News here.














Comments
0 comment