views
X
Research source
If you have a few cold temperature experiments in mind, cryogenic-temperature alcohol might be perfect.
Making the Cryogenic-Temperature Alcohol

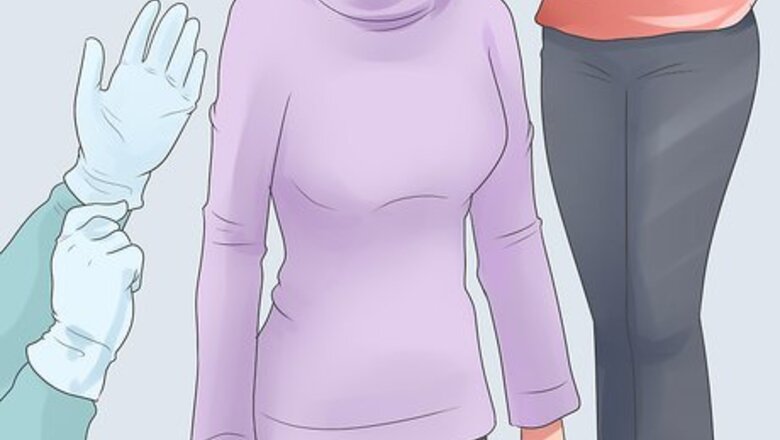
Dress appropriately. Wear long pants, a long-sleeve shirt, and sturdy work gloves. You should also wear protective goggles and consider tying back your hair if it's long. While this may seem excessive, cryogenic-temperature alcohol is highly flammable, can cause dizziness and irritate your skin. Your workspace should be free from food and drink. It should also be well ventilated and away from hot surfaces or open flame.
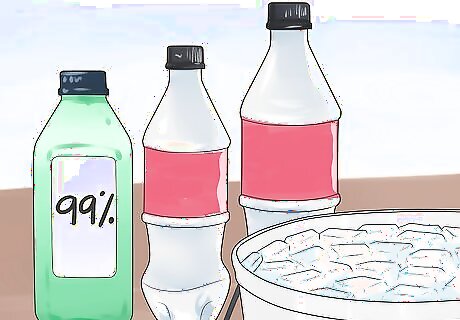
Gather your supplies. You'll need a 2 liter (0.5 US gal) soda bottle, a smaller plastic bottle (such as a smaller soda bottle) that can fit inside the larger soda bottle, scissors, 99% isopropyl alcohol, and pellets of dry ice. Both bottles should be empty, clean, and dry. If you remove the labels, you'll be able to watch the creation of cryogenic-temperature alcohol.
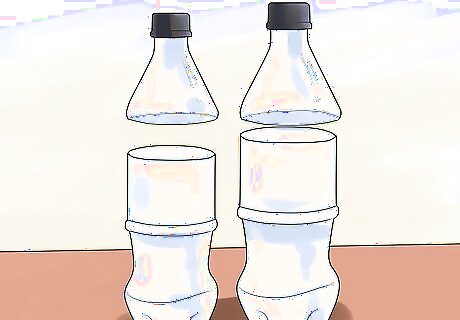
Prepare your bottles. Use a pair of sharp scissors to cut approximately 3 inches (7.62 centimeters) off the top of both bottles. Recycle or discard the tops. Make sure the smaller bottle can easily fit in the larger bottle.
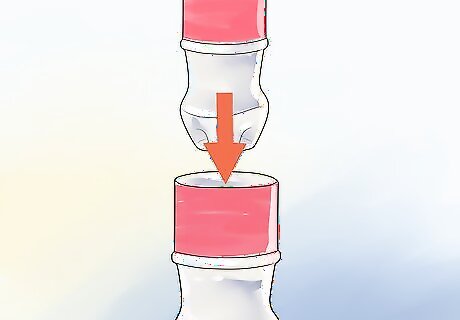
Nest your bottles. First, you'll want to use scissors to poke holes around the bottom and lower sides of the small bottle. Then, set it inside the larger one.
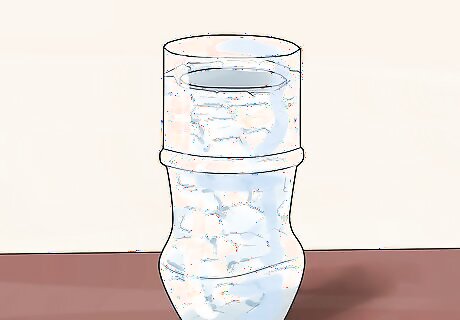
Add the dry ice pellets. Evenly distribute them along the inside of the 2 liter (0.5 US gal) bottle while you hold the smaller empty bottle in the center. This will keep it level. If you don't have pellets, you can chip your own dry ice. Just be careful using a knife and chip the dry ice into 1/2" chunks. Always wear gloves when handling dry ice, as it can cause injure bare skin.
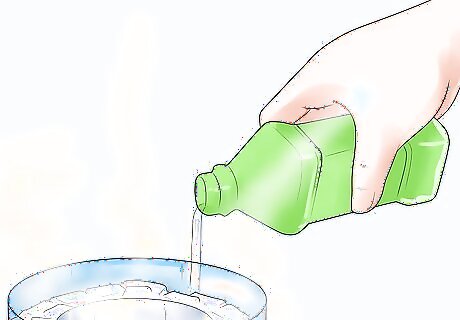
Pour in the isopropyl alcohol about 2" deep. Slowly pour the alcohol directly over the dry ice pellets. You may want to gradually turn the bottle as you pour, since the dry ice will start fogging, making it difficult to see. If you use a lower percentage isopropyl alcohol, it will freeze in a thick gel. Remember to avoid touching the cryogenic-temperature alcohol, which will stick to your hands.

Wait till the liquid stops bubbling. Once the dry ice has stopped fogging, you should be able to see that your smaller bottle now has several inches of the clear cryogenic-temperature alcohol in it. You can now begin using it in experiments. Your liquid is now at its lowest temperature. Use extreme care when handling.
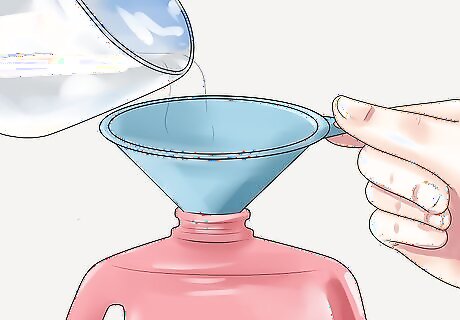
Pour the liquid nitrogen into a sturdy container and label it appropriately. It can be kept at room temperature for future use for up to 30 days. Then, discard the isopropyl alcohol according to local regulations. Do not inhale, touch with bare skin, or consume the cryogenic-temperature alcohol. If it gets in your eyes or on your skin, rinse repeatedly with water. If inhaled, move to fresh air and rest. Call a poison control center if you feel unwell.
Using Your Cryogenic-Temperature Alcohol
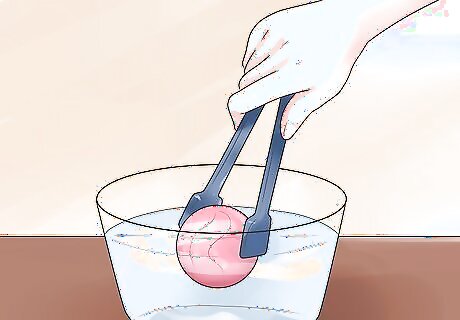
Try freezing items. This is a simple experiment. Use tongs to dip items into the cryogenic-temperature alcohol until they become hard. Remove the items and shatter them, if you like. Flowers, leaves, fruits, vegetables, and small rubber balls are just a few of the things you can freeze and shatter using cryogenic-temperature alcohol. Do not eat them and remember to wear gloves when handling.

Dip a small balloon to make "liquid air." Use a small balloon that you can nearly fit into your container of cryogenic-temperature alcohol. While wearing gloves, dip most of the balloon into the liquid. The balloon will start to shrink and you should notice liquid inside the balloon. To return the "liquid air" inside the balloon back into a gas, simply place your balloon in a warm area and wait for the particles to move faster and expand.
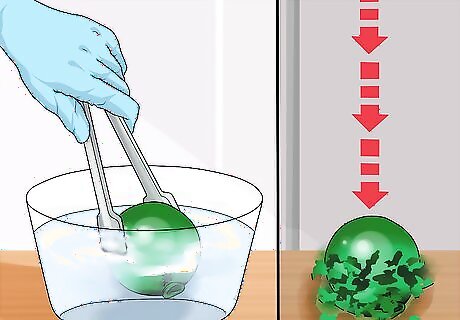
Shatter a ball. Roll modeling clay into a ball and dip it into the cryogenic-temperature alcohol. Drop it on the floor or another hard surface and watch it shatter.

Research potential experiments. If you've found an experiment that uses liquid nitrogen, consider whether or not it will work with cryogenic-temperature alcohol. While the liquid nitrogen creates nitrogen gas, the cryogenic-temperature alcohol does not. Choose experiments that simply use the liquid nitrogen for its temperature-lowering aspect. Never eat any experiments that you've done with food and cryogenic-temperature alcohol.


















Comments
0 comment